Join us at Medica from 17th to 20th November
Discover our product range and distributor opportunities
(Hall 16 - pod 35)
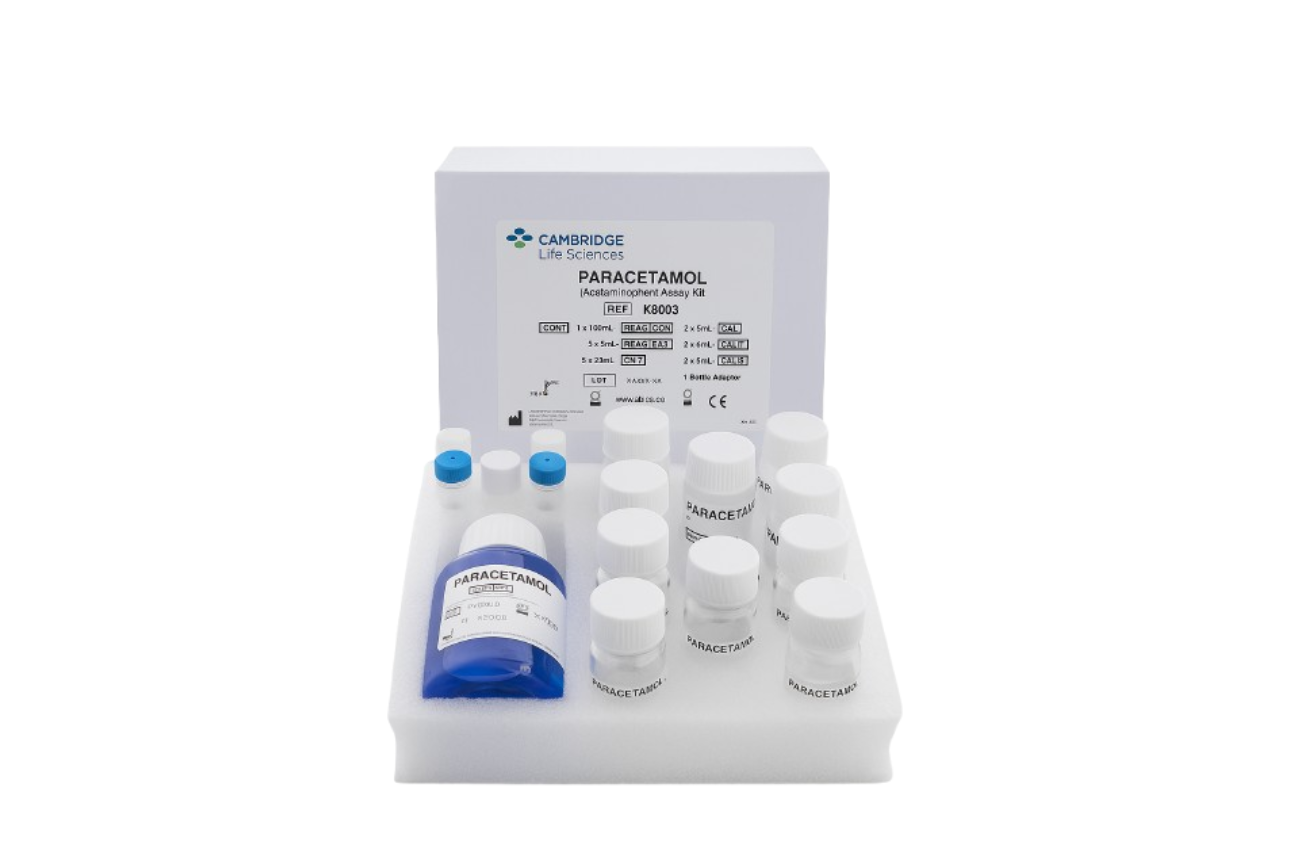
Accurate, rapid assays for Paracetamol and Salicylate level detection
Therapeutic Drug Monitoring
Our Therapeutic Drug Monitoring range provides clinicians and laboratories with high-quality diagnostic tools to ensure safe and effective patient care. Designed for accuracy, speed, and compatibility with major clinical chemistry analysers, these assays support rapid diagnosis in cases of potential overdose and routine therapeutic monitoring.
All products are manufactured in the UK under strict ISO-certified quality systems and come with clear, validated instructions for consistent performance across laboratories.
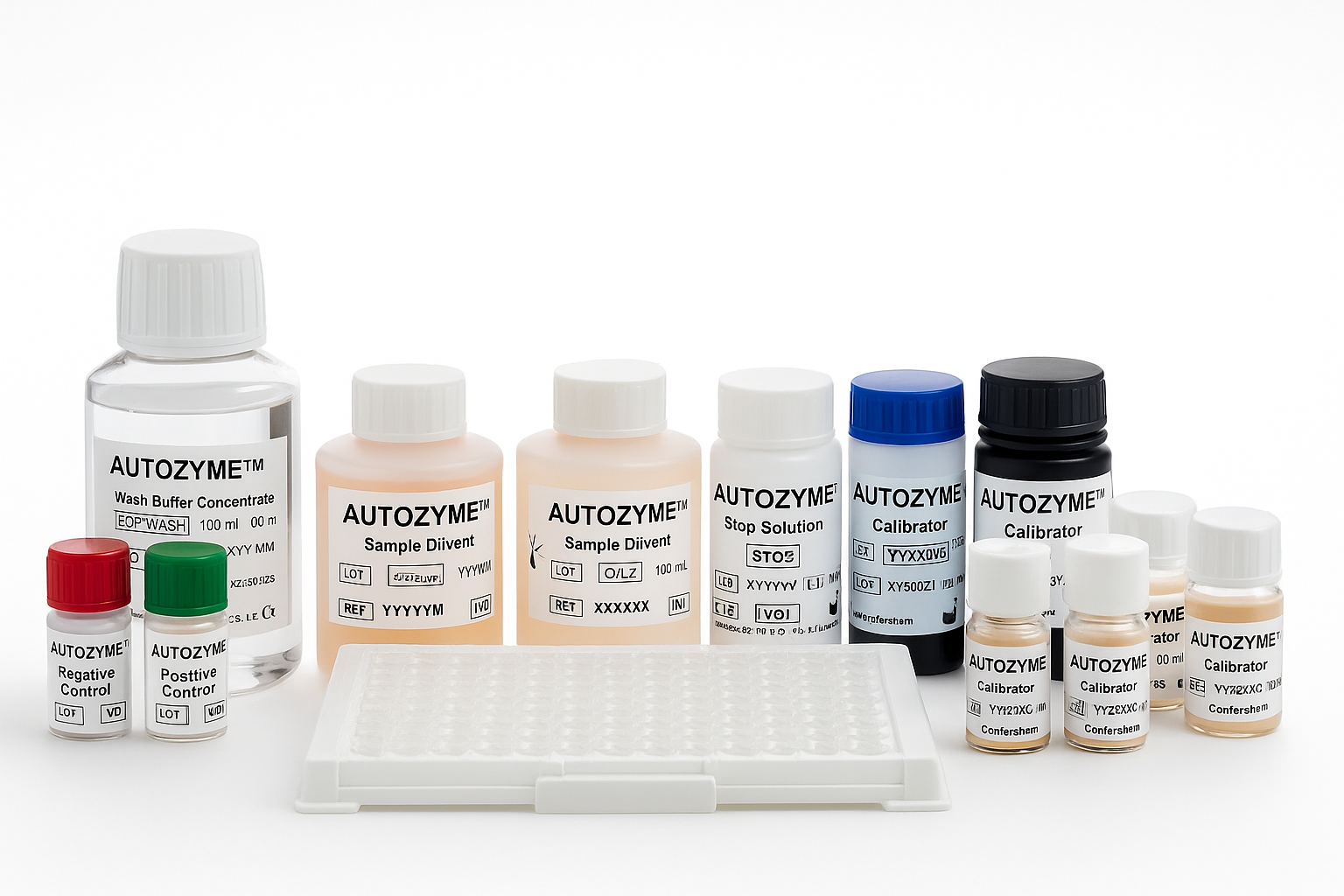
Detects autoantibodies across all three isotypes (IgA, IgM, IgG)
Autoimmune Diagnostics
Our AUTOZYME ELISA range provides highly sensitive and specific assays to support the diagnosis and monitoring of autoimmune diseases.
Developed in-house under ISO-certified quality systems, these assays are trusted by laboratories worldwide for their reliability, consistency, and compatibility with standard ELISA platforms.
Each kit comes with clear, validated instructions to ensure accurate and reproducible results across laboratories.

Fast, reliable detection of Helicobacter pylori during endoscopy procedures
Rapid Helicobacter Pylori Test
The Endosc-HP® Rapid Urease Test is an in vitro diagnostic assay for quick detection of Helicobacter pylori from gastric biopsy samples during endoscopy. Results are available within minutes, enabling immediate clinical decisions without complex equipment. The twin-well cartridge contains urea, phenol red, and buffer salts; the presence of H. pylori urease causes a color change from yellow to magenta. Trusted worldwide for its accuracy and ease of use, the test is room temperature–stable and UK-manufactured under ISO-certified standards. Product Code U1021 includes four ready-to-use tests with clear instructions for consistent performance.

Cambridge Life Sciences
Proudly Associated With BIVDA
BIVDA is the UK's leading IVD industry association, representing 97% of the industry...
UK NEQAS | BIVDA | LRQA CERTIFIED | ISO 13485 | UKAS MANAGEMENT SYSTEMS |

Certifications, Accreditations & Partners
ISO 13485:2016 certified
- LRQA
- UKCA
We proudly partner with:
- The UK National External Quality Assessment Service (UK NEQAS)
- The British in Vitro Diagnostics Association (BIVDA)

1985 – Established in Cambridge
Founding & Early Years
Cambridge Life Sciences began its journey in Cambridge Business Park, pioneering development of in-vitro diagnostic assays. Driven by a vision to support clinicians and labs with fast, accurate tools, Cambridge Life Sciences laid its foundation in rigorous science and innovation.

1989 – Expansion to Ely, Cambridgeshire
Growth & Relocation
As demand grew, Cambridge Life Sciences relocated and expanded its operations to Ely. This move allowed for enhanced manufacturing capacity and closer integration of R&D, test development, and quality control — accelerating growth and reinforcing its reputation in diagnostics.
2021 – Expanded to a group partnership
Strategic Partnerships & Distribution Shift
In 2021, Cambridge Life Sciences became part of larger group, embedding its diagnostic strengths within a broader healthcare network.
2023 - Collaboration with market leaders
PARTNERSHIP & POTENTIAL
From 1st February 2023, Cambridge Life Sciences partnered with MedScience Distribution Ltd to take over all UK distribution, support, and customer service — ensuring stronger reach, infrastructure and service in the UK market.

2025 – associates of MedScience Group
Present & Tomorrow
Now fully positioned alongside MedScience Distribution, Cambridge Life Sciences continues to operate as a trusted manufacturer and innovator in diagnostics. With expanded support, logistics, and sales capabilities from MedScience, Cambridge Life Sciences is geared towards global growth — all while maintaining excellence in diagnostics.
Excellence in Diagnostics — Trusted worldwide for innovative in vitro diagnostic solutions, all UK-manufactured under ISO 13485:2016